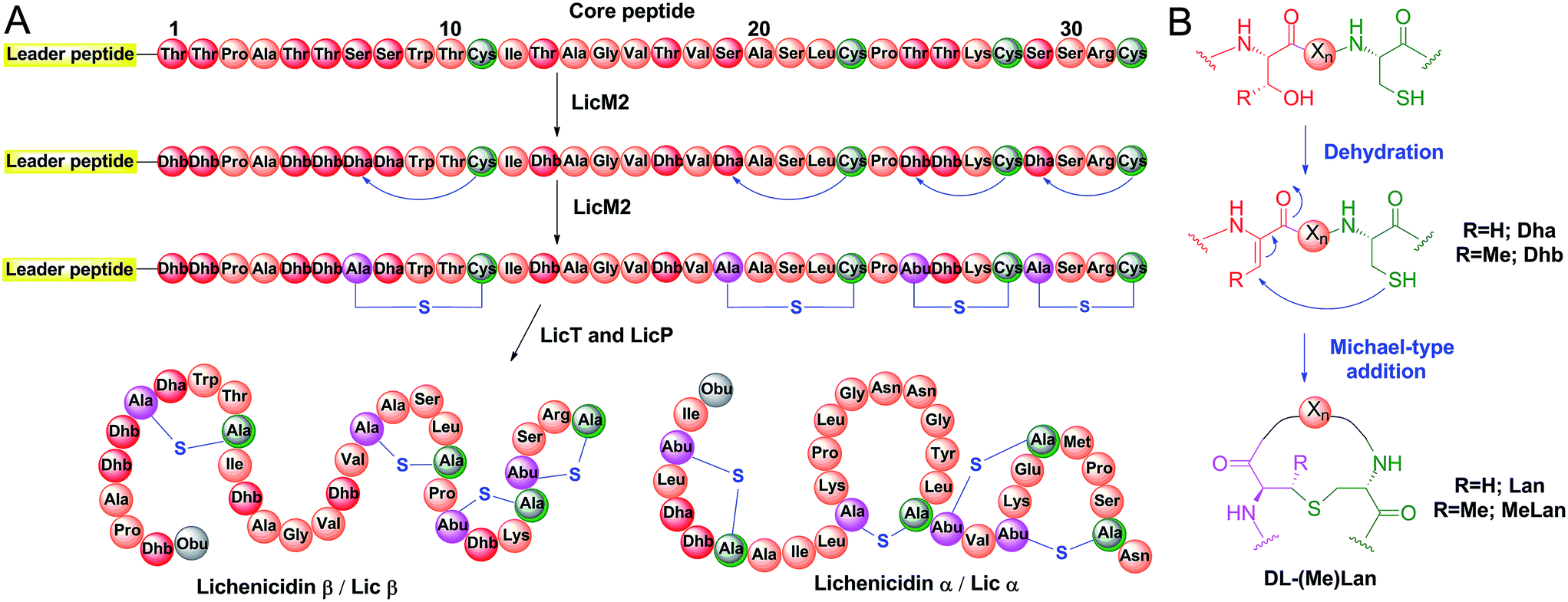
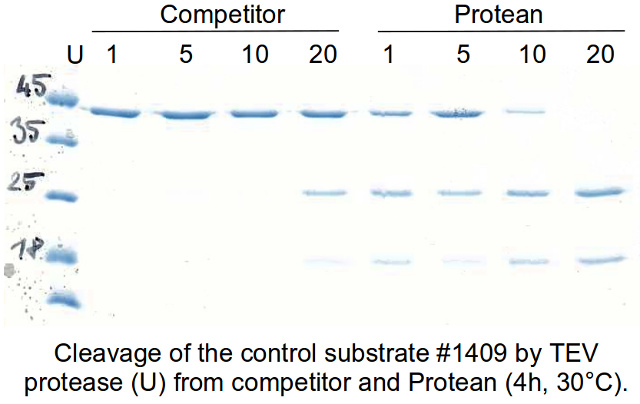
TEV protease cleavage of bioGATA-2 bound to streptavidin beads. (A)... | Download Scientific Diagram

Phosphorylation regulates proteolytic efficiency of TEV protease detected by a 5(6)-carboxyfluorescein-pyrene based fluorescent sensor - ScienceDirect

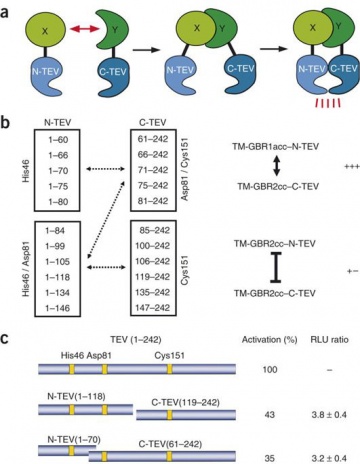
A split protease-E. coli ClpXP system quantifies protein–protein interactions in Escherichia coli cells | Communications Biology

Engineering of TEV protease variants by yeast ER sequestration screening (YESS) of combinatorial libraries | PNAS

YESS 2.0, a Tunable Platform for Enzyme Evolution, Yields Highly Active TEV Protease Variants | ACS Synthetic Biology

YESS 2.0, a Tunable Platform for Enzyme Evolution, Yields Highly Active TEV Protease Variants | ACS Synthetic Biology
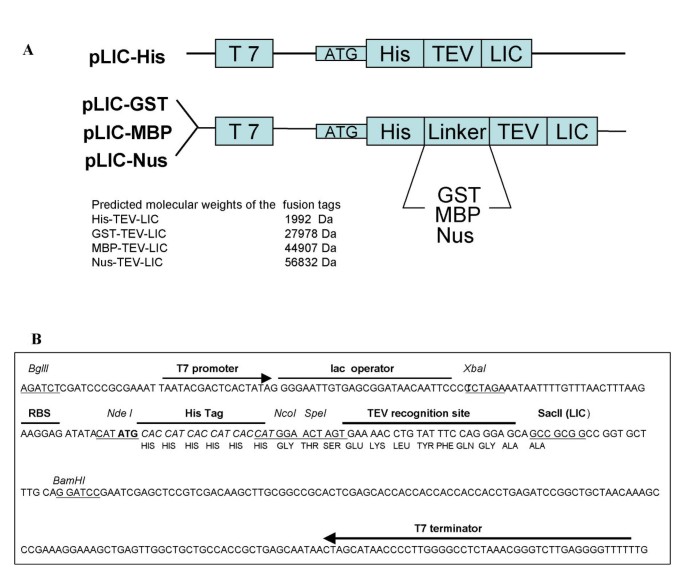
A family of E. coliexpression vectors for laboratory scale and high throughput soluble protein production | BMC Biotechnology | Full Text

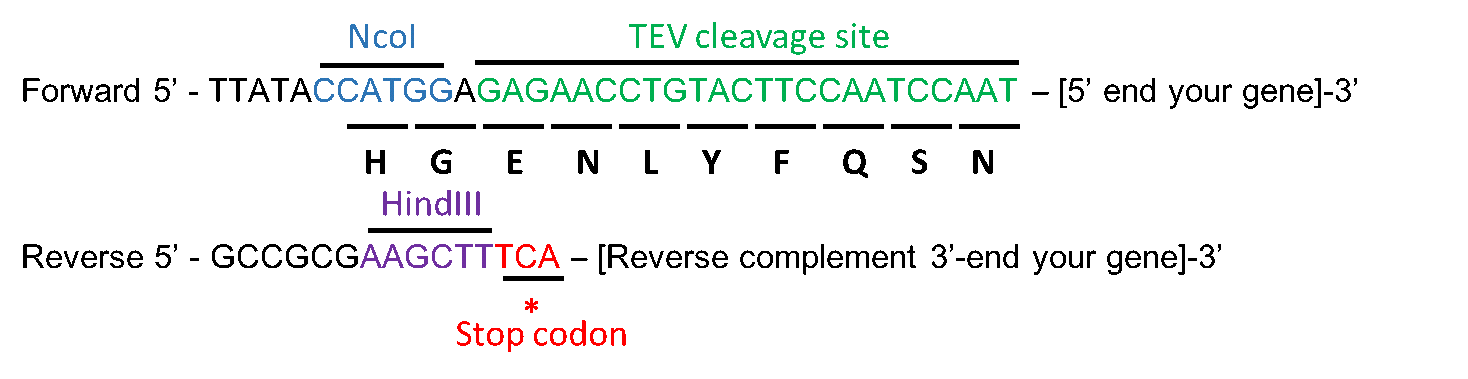
Improved yield, stability, and cleavage reaction of a novel tobacco etch virus protease mutant | SpringerLink

Partial map of the pET28a-TEV plasmid. A) DNA sequence of pET28a-TEV... | Download Scientific Diagram
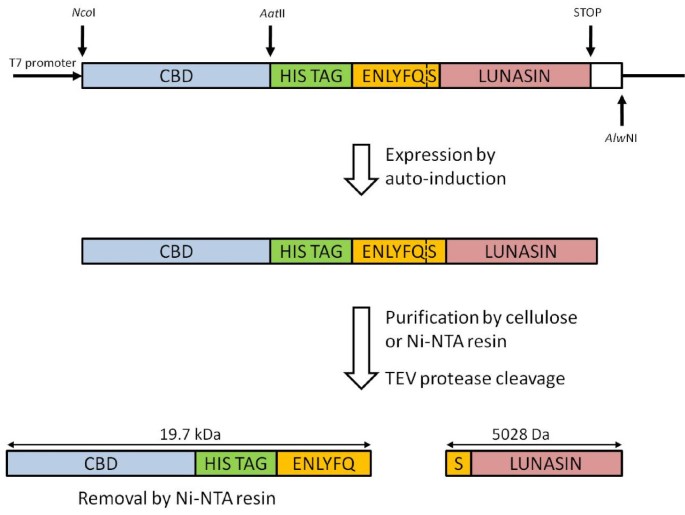
Highly efficient soluble expression, purification and characterization of recombinant Aβ42 from Escherichia coli