Write an equation for the reaction of p-bromobenzaldeyde with HCN (NaOH as the catalyst). | Homework.Study.com

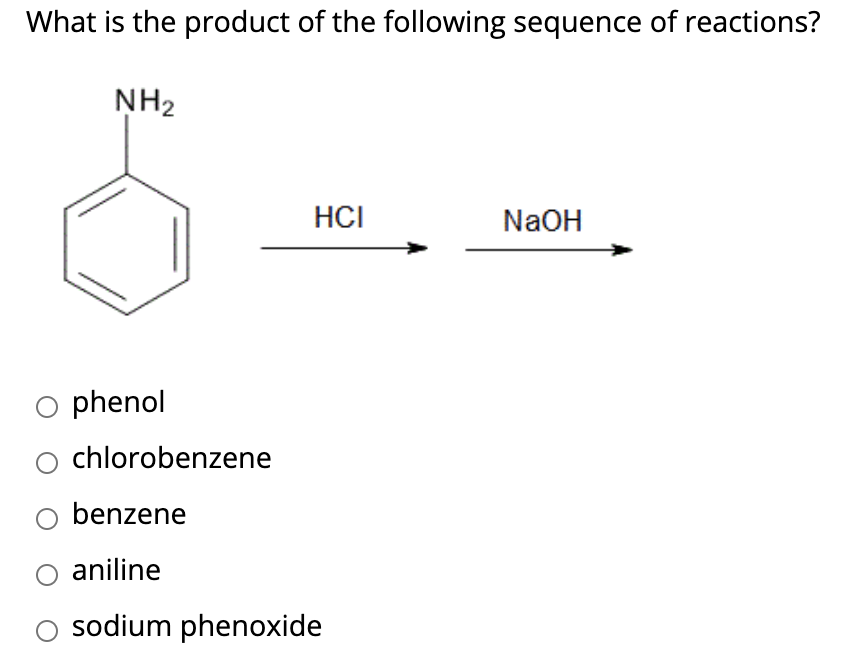
Write the Product Formed When P-nitro Chlorobenzene is Heated with Aqueous Naoh at 443k Followed by Acidification? - Chemistry | Shaalaa.com

Predict the product of the reaction of p-methylbenzoic acid with the stated reagent. NaOH, then CH3I | Homework.Study.com

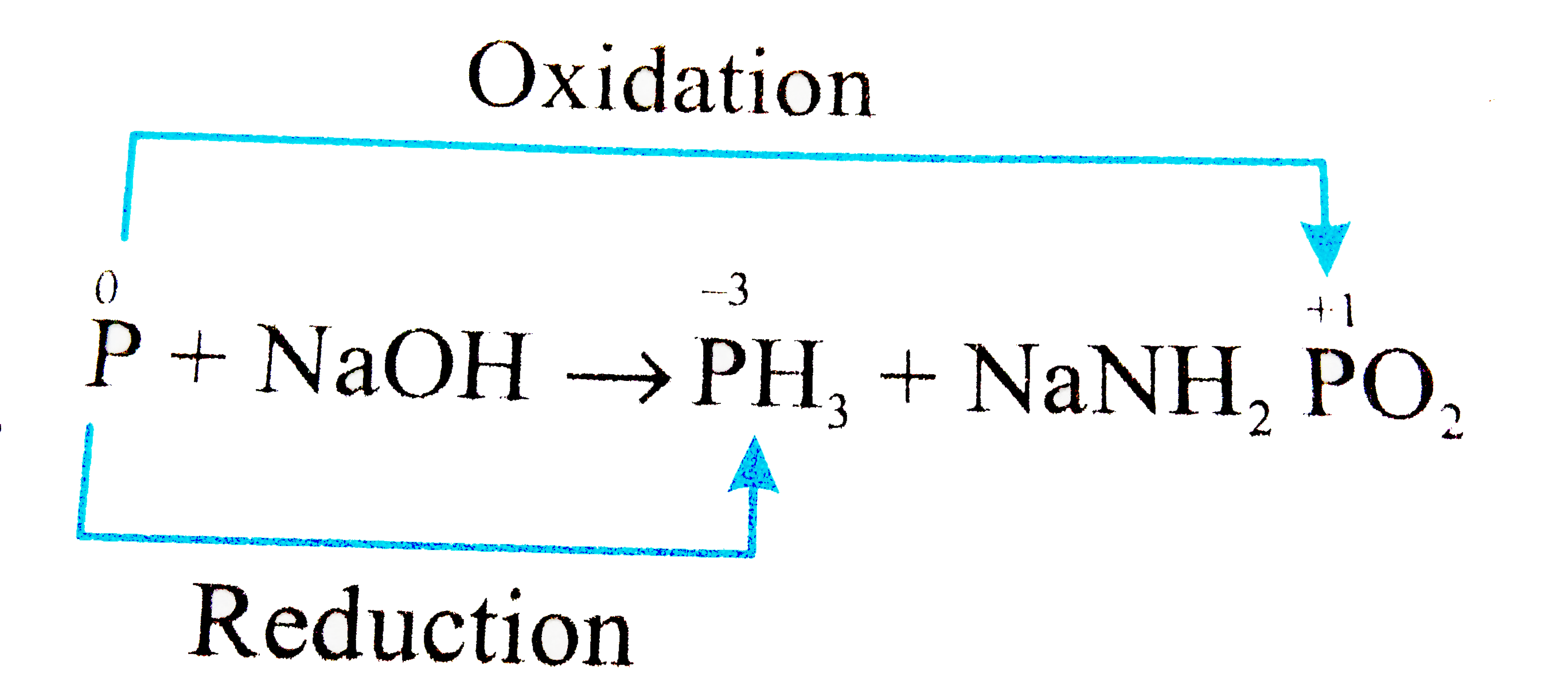
The reaction of white phosphorus with aqueous NaOH gives phosphine along with another phosphorus containing compound. The reaction type, the oxidation states of phosphorus in phosphine and the other product are respectively:
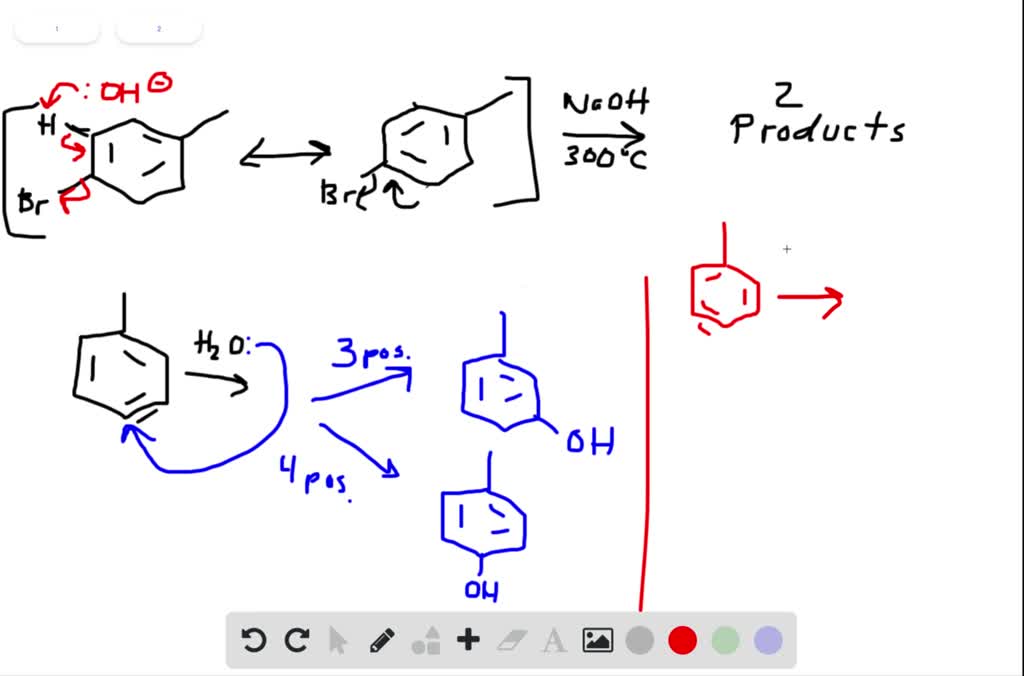
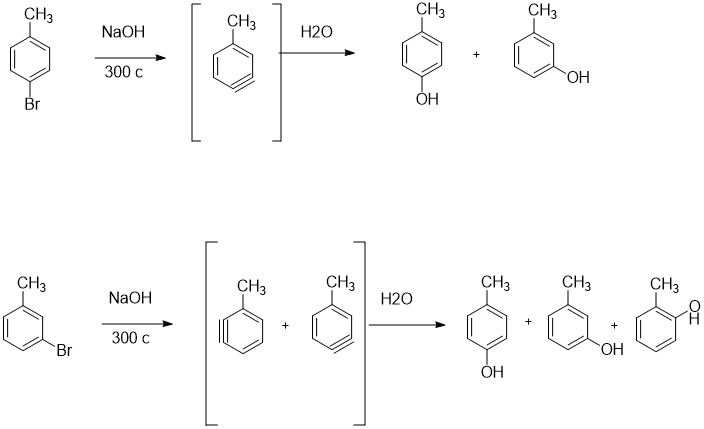
SOLVED:Treatment of p -bromotoluene with NaOH at 300^∘ C yields a mixture of two products, but treatment of m -bromotoluene with NaOH yields a mixture of three products. Explain.
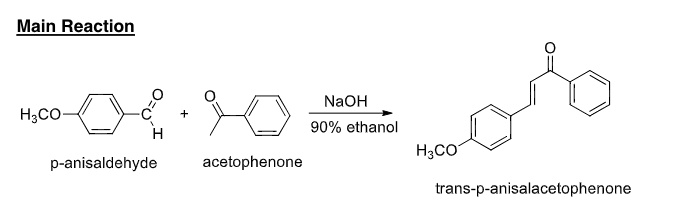
SOLVED: Main Reaction NaOH 90% ethanol HzCO acetophenone trans-p-anisalacetophenone HzCo p-anisaldehyde

Scheme 1. (i) and (iii) NaOH/p-toluenesulfonyl chloride, (ii) K 2 CO 3... | Download Scientific Diagram
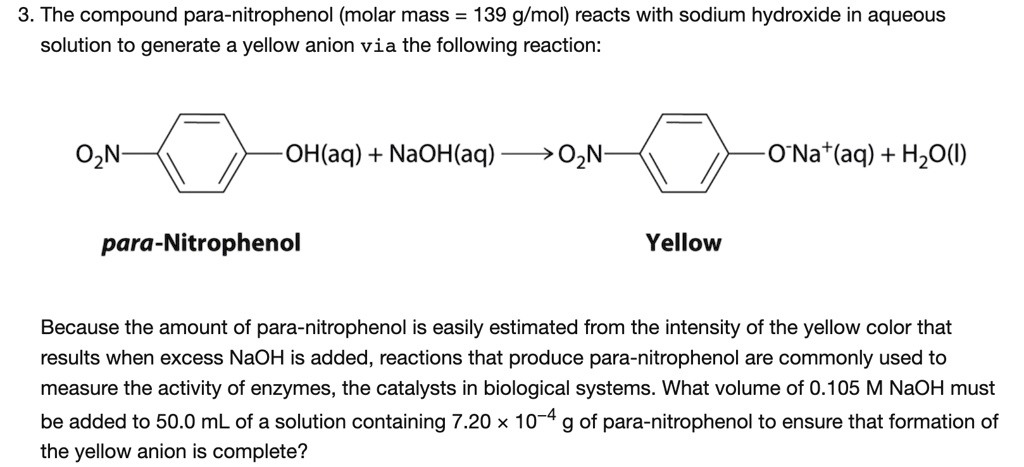
SOLVED: 3. The compound para-nitrophenol (molar mass = 139 g/mol) reacts with sodium hydroxide in aqueous solution to generate a yellow anion via the following reaction: OzN OH(aq) + NaOH(aq) O2N 0

Cell Wash Solution I /Naoh-D 2L Roche Reagent Modular P/D Cobas C702 - China Cell Wash Solution I /Naoh-D and Roche Reagent

Immobilization and characterization of Fe(0) catalyst on NaOH-treated coal fly ash for catalytic reduction of p-nitrophenol - ScienceDirect